Abstract
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of Non-Hodgkin Lymphoma, with approximately 25-30% of patients presenting as early or limited-stage (stage I and II). Though treatment with chemoimmunotherapy is standard of care, the role of consolidative radiotherapy (RT) in limited-stage (LS) patients is not well defined. Recent studies have attempted to shorten chemotherapy courses and eliminate RT for LS patients. Additionally, recent single institutional data has suggested LS patients with extranodal (EN) disease may have better outcomes if treated with RT. We aimed to investigate whether the addition of RT provided a survival benefit in early-stage DLBCL patients based on primary site at presentation comparing nodal to EN disease.
Methods
The National Cancer Database was utilized to identify patients diagnosed with LS-DLBCL from 2004 to 2015. Only patients treated with multiagent chemotherapy were included. Patients with international prognostic index (IPI) score available were separated into low (0-1 factors) or high-risk (2-4) groups. Landmark analysis was performed to exclude patients with last contact, including death, within 12 months of diagnosis. Kaplan-Meier survival analysis was used to compare overall survival (OS). Cox regression analysis was used to identify hazard ratios for survival using RT between subgroups.
Results
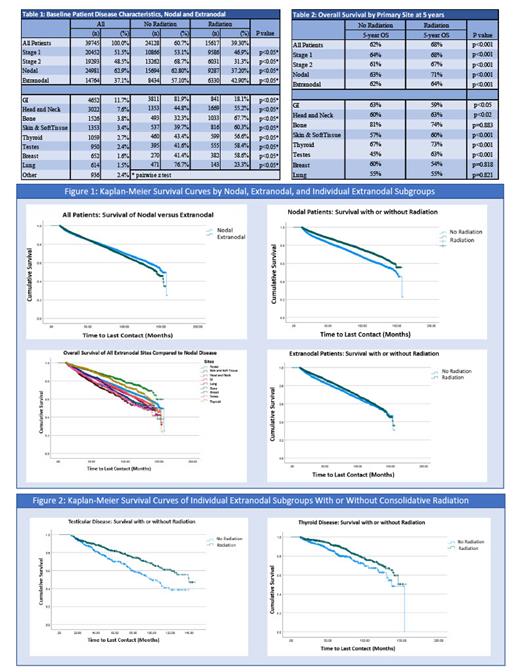
Of the 39,745 LS patients identified, 62.9% had nodal disease and 37.1% had EN disease with stage I disease accounting for 51.5% of patients. Only 6,628 patients had reported IPI scores with the majority having low-risk IPI (69.2%). Compared to patients with only nodal disease, patients with EN involvement were more likely to receive consolidative RT (42.9% vs 37.2%; p<0.05). EN patients were most likely to receive RT with primary bone (67.7% of 1526 patients), skin/soft tissue (60.3% of 1353), breast (58.6% of 652), testes (58.4% of 950) and thyroid (56.6% of 1059) involvement (all p<0.05). GI (18.1% of 4652) and lung (23.3% of 614) primaries were least likely to receive RT (p<0.05). With a median follow up of 58.8 months, the addition of RT was associated with improved 5-year OS for all LS patients as compared to those treated with chemotherapy alone (68 vs. 62%, p<0.001). While RT was associated with improved 5-year OS in both the nodal and EN disease patients, nodal patients had a greater benefit of receiving RT as compared to EN patients (nodal: 71% vs. 63%, p<0.001; EN: 64% vs. 62%; p<0.001). Specifically, in EN patients, the addition of RT significantly increased 5-year OS for skin/soft tissue (60% vs. 57%, p<.001), head and neck (63% vs. 60%, p<0.02), testicular (63% vs. 45%, p<.001), and thyroid sites (73% vs. 67%, p <0.02). Furthermore, RT slightly improved 5-year OS outcomes for patients with both low-risk (75% vs. 73%; p<0.01) and high-risk IPI (52% vs. 50%; p<0.04). On multivariate analysis, adjusting for age, stage, and Charlson-Deyo comorbidity score, the addition of RT was an independent factor for improved survival for all LS patients (Hazard Ratio [HR] 0.84, 95% Confidence Interval [CI] 0.81-0.88; p<0.001) along with EN sites of skin/soft tissue (HR 0.72, 95% CI 0.58-0.88; p<0.01), testicle (HR 0.70, 95% CI 0.54-0.90, p<0.01) and thyroid (HR 0.68, 95% CI 0.51-0.90; p<0.01). No significant survival differences were observed in patients with EN involvement of the lung, bone, breast, or GI tract when RT was added to their treatment.
Conclusions
We report the largest retrospective study on EN outcomes utilizing consolidative RT in early-stage DLBCL. Though there is no consensus on optimal treatment indications for RT in LS-DLBCL, this data shows improved OS in both nodal only disease and within specific EN disease subgroups when RT is added to front-line chemotherapy. As recent studies have attempted to shorten chemotherapy cycles and limit the use of RT, these results suggest caution against omission of RT in select patients.
Shah: AstraZeneca: Research Funding; Seattle Genetics: Research Funding; Epizyme: Research Funding. Stephens: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Research Funding; Abbvie: Consultancy; JUNO: Research Funding; Innate Pharma: Membership on an entity's Board of Directors or advisory committees; Adaptive: Membership on an entity's Board of Directors or advisory committees; Arqule: Research Funding; Mingsight: Research Funding; Beigene: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy; CSL Behring: Consultancy; Celgene: Consultancy; Karyopharm: Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal